 by Chee-Leok Goh, MD, MBBS, MRCP(UK), MMed(Int Med);
by Chee-Leok Goh, MD, MBBS, MRCP(UK), MMed(Int Med);
Sai Yee Chuah, MBChB (Glasgow), MRCP(UK), Dip Derm, MRCPS (Glasgow); Steven Tien, MD; Guan Thng, MBBS, MRCP; María Alejandra Vitale, MD; and Arancha Delgado-Rubin, PhD
Drs. Goh, Chuah, and Thng are with the National Skin Centre in Singapore. Drs. Vitale and Delgado are with the R&D Department at Industrial Farmacéutica Cantabria (IFC), SA in Madrid, Spain.
Funding: This study was sponsored by Industrial Farmacéutica Cantabria (IFC), SA.
Trial Registry: NHG Domain Specific Review Board (DSRB); Trial Registration Number: 2013/00237
Disclosures: Drs. Goh, Chuah, and Thng report no conflicts of interest to relevant to the content of this article. Drs. Vitale and Delgado are employees of Industrial Farmacéutica Cantabria (IFC), SA.
Abstract: Melasma is a common pigmentary disorder with a multifactorial etiology that can hinder its management. The aqueous extract of the fern Polypodium leucotomos (PLE), Fernblock® (IFC, Madrid, Spain), has demonstrated antioxidant and photoprotective activities and has been used for the treatment of several pigmentary disorders. The aim of this study was to evaluate the efficacy and safety of oral PLE in the treatment of melasma in Asian patients. Forty healthy adult patients with clinical diagnoses of melasma who were receiving treatment with topical 4% hydroquinone cream and sunscreen with a sun protection factor (SPF) of 50+ were recruited for inclusion in this study from the National Skin Centre in Singapore. They were randomized to receive either oral PLE supplementation or placebo for 12 weeks. Patients were assessed at baseline, Day 28, Day 56, and Day 84 using the modified Melasma Area and Severity Index (mMASI); melanin and erythema indexes; VISIA® photography (Canfield Scientific, Parsippany, New Jersey, USA); and the Melasma Quality of Life (MelasQoL) questionnaire. Adverse events were recorded. Following four, eight, and 12 weeks of treatment, there were statistically significant differences between the mMASI scores of both groups as compared with the baseline scores (p?0.01). mMASI scores of the PLE group at eight and 12 weeks were also significantly lower than those of the placebo group (p?0.05). At the end of the study, a significant improvement was reached in both groups (both p?0.01), with no significant differences between them. The scores of the melanin and erythema indices displayed a slight improvement in both groups, without significant differences among them. MelasQoL score showed an improvement in the PLE group versus the placebo group. Our results demonstrate that the PLE aqueous extract product significantly improves and accelerates the outcome reached with hydroquinone and sunscreen almost from the first month of treatment in comparison with the placebo. There were no significant side effects reported. The oral PLE aqueous extract product appears to be a safe and effective adjunctive treatment for melasma in combination with topical hydroquinone and sunscreen.
Keywords: Choasma, pigmentation, antioxidant, photoprotection, fern plant extract
J Clin Aesthet Dermatol. 2018;11(3):14–19
Melasma is a common acquired benign pigmentary disorder that occurs on sun-exposed areas of the skin. It is especially common among Asian and Hispanic individuals1 and boasts a considerable impact on the psychosocial wellbeing of affected individuals.2,3 Its etiology is multifactorial, including sunlight exposure, pregnancy, and contraceptive pills, as well as hormone replacement therapy, making its treatment challenging.
The gold standard treatment for melasma is topical hydroquinone cream and broad-spectrum sunscreens. However, hydroquinone has been associated with the development of exogenous ochronosis4,5 and contact allergy. Several treatment options, including oral tranexamic acid, chemical peels, intense pulsed light (IPL) or laser therapy, and topical products with different depigmenting ingredients (e.g., retinoids, kojic acid, licorice extract, ruscinol, resveratrol) have been used in different combinations with variable degree of success and complications.6,7
Polypodium leucotomos (PL) is a fern from the Polypodiaceae family, which is native to Central and South America. Fernblock® (IFC, Madrid, Spain), an aqueous extract of PL (PLE), is a potent antioxidant ingredient8 with demonstrated photo- and immunoprotective activities against ultraviolet (UV) A and UVB radiations.9,10 It is available in over-the-counter formulations in topical and oral sunscreens and has been used since the 1970s for the treatment or adjunctive treatment of various skin conditions, including psoriasis, atopic dermatitis, vitiligo, and polymorphous light eruption.11 Studies have found it can significantly reduce the severity of sunburn,12 decrease the risk of UV radiation-induced skin cancer, and prevent skin aging.13 The reported mechanisms of action include decreasing UV-mediated oxidative damage to deoxyribonucleic acid (DNA), enhancing the activity of endogenous antioxidant systems, increasing the minimal erythema dose (MED), blocking UV radiation-induced cyclooxygenase-2 expression, reducing UV-induced immune suppression, and promoting p53 suppressor gene expression.14
The potential side effects of oral PLE are mild. A recent meta-analysis assessing 40 years of clinical and preclinical studies found that oral PLE was administered at daily doses ranging from 120mg to 1,080mg.15 Only two percent of the patients (16/1,016) reported mild gastrointestinal symptoms or pruritus; no serious adverse effects were reported. Long-term safety was assessed by Murbach et al,16 who showed the complete absence of toxicity in a 90-day study conducted in male and female Wistar rats that received up to 1,200mg/kg body weight/day. Furthermore, a clinical study involving healthy adult subjects demonstrated that 240mg of PLE taken twice daily for 60 days was a safe and effective means of reducing the damaging effects of UV radiation.17
Beneficial effects of PLE have also been demonstrated in pigmentary disorders. Schalka et al18 reported that oral intake of PLE for seven, 14, or 28 days increased the mean values of minimum pigmentary and erythema doses of volunteers exposed to UVA and UVB radiation, demonstrating that the continued use of PLE is effective in increasing individual resistance to UV-induced pigmentation and erythema and thus might be used to treat pigmentary disorders. Furthermore, two double-blind, placebo-controlled studies19,20 demonstrated the clinical efficacy of PLE as an adjunct to sunscreen for the treatment of melasma in 54 female subjects who received PLE daily for 12 weeks. In a recent review,12 researchers concluded that orally administered PLE also appears to provide benefits in treating vitiligo and melasma and might help with post-inflammatory hyperpigmentation.
Our study was carried out to determine the efficacy and safety of oral PLE for the treatment of melasma in an Asian population. We hypothesized that the combination of PLE with topical hydroquinone 4% cream would be more effective than the combination of topical hydroquinone 4% and sunscreen with sun protection factor (SPF) 50 alone.
Population and Methods
This was a single-center, prospective, randomized, double-blind, placebo-controlled trial that compared oral PLE and a placebo in the treatment of melasma. The protocol used in this study adhered to the Good Clinical Practice guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use and was approved by the hospital’s institutional review board. All subjects provided informed consent prior to undergoing any study-related procedures. Subjects pictured in this article provided photoconsent.
Subjects. Forty healthy adult patients with clinical diagnoses of melasma who were being treated with the standard topical treatment, i.e. topical 4% hydroquinone cream and sunscreen SPF 50+, were recruited at the National Skin Centre (NSC) in Singapore over a six-month period. Patients were identified by a member of the study team. For women of childbearing age, a urine pregnancy test was performed prior to recruitment.
Inclusion and exclusion criteria. Subjects aged 25 to 65 years with clinical diagnoses of facial melasma and Fitzpatrick Skin Types III or IV were selected. Patients were excluded if they were pregnant, lactating, or participating in other clinical studies, or if they were known to have contact allergy to any of the ingredients in the topical medication used or a sensitivity to oral PLE.
Randomization. Randomization was carried out via a computer-generated randomization list created by a statistician. The list was then sent to an independent pharmacist to be used for the preparation and labeling of the study drugs. The randomization list was kept secure until the end of study and was accessible only to the statistician and the pharmacist. Unblinding of the study occurred only after all of the patients had completed the study.
Treatment regimen. Subjects were randomly assigned in a 1:1 ratio to receive either the oral PLE product or a placebo twice daily (total daily dose of 480mg) at 8am and 1pm for a total of 12 weeks. The recommended dose of PLE is 2 to 4 capsules (240–480mg) per day in two divided doses. In our study, we chose the higher treatment dose of two capsules (480mg) twice daily in order for the PLE to confer more effective sun protection, as the study was conducted in an equatorial country that boasts intense sunlight throughout the year. In addition, all of the subjects continued with their prescribed topical 4% hydroquinone cream at night and sunscreen SPF 50+ during the daytime throughout the study period. Patients were asked to stop all other facial topical creams except for their regular cleansers. The study product contained 240mg of PLE per capsule, while the placebo was an inert capsule of similar appearance provided by the sponsor company. Both PLE product and placebo were packed in identical containers, labeled with the trial number, and stored at room temperature. An adequate supply of the study capsules was dispensed at each visit.
Assessments. Information on patient demographics, past medical history, dermatological history, drug and contact allergies, hours spent outdoors per day, use of skin-lightening agents, use of lasers or light therapies, and skin care routine were recorded. The subjects were assessed during four different visits: at baseline (V1), on Day 28 (V2), on Day 56 (V3), and on Day 84 (V4). At each visit, they were assessed using the Melasma Area and Severity Index (mMASI).21 A fixed area of the skin deemed by the investigator to be the darkest on each patient was assessed for pigmentation and erythema using a skin colorimeter (Mexameter™, Courage + Khazaka electronic GmbH, Cologne, Germany) at each visit. An average of three readings was taken and the mean was calculated. Iconography was obtained by VISIA photography (Canfield Scientific, Parsippany, New Jersey, USA), and independent dermatologists assessed the clinical photographs taken at each visit for degree of involvement. A Melasma Quality of Life (MelasQoL) questionnaire was performed to evaluate the quality of life of the subjects at each time point.22 Patients were also asked about any adverse effects at each visit.
Statistical analysis. Summary statistics were reported for patient characteristics. An analysis of the absolute values and variation in outcome measurements, such as mMASI, colorimeter melanin and erythema scores, and MelasQoL, was performed using a simple paired t-test for normal-distributed data; otherwise, a non-parametric Wilcoxon signed-rank test was used. The null hypothesis was that the means at every time point would be equal. Graphs show the standard error of the mean. For a comparison of the changes in outcome measurements between the treatment and placebo groups, the selected test was the Mann–Whitney U nonparametric test. A p value of less than 0.05 was considered to be statistically significant.
Primary efficacy variable. The primary efficacy variable was the reduction in mMASI score between the baseline and the end of the study. mMASI score is a universally accepted scoring system for quantifying the severity and extent of melasma. At each visit, the study team member assessed the area of involvement and level of darkness at the forehead, right and left malar regions, and the chin.21
Secondary efficacy variables. The secondary efficacy variables were the reduction in melanin and erythema indexes as measured by the skin colorimeter. Additionally, subjective improvement of melasma was assessed by patient completion of the MelasQoL questionnaire at each visit and dermatologist assessment of the clinical photographs.
Safety and tolerability. Side effects were recorded throughout the study.
Results
Forty patients (37 women and 3 men) aged between 37 and 65 years of age (mean ± standard deviation [SD] : 50.9±5.1) with Fitzpatrick Skin Types III or IV were enrolled. During the study, five subjects dropped out, one of whom was male. The other two men included one in the placebo group and the other in the active group, respectively. Thus, in order to homogenize the sample, these two men were excluded from the statistical analysis, resulting in a final sample of 33 evaluated female subjects. The patients were predominantly Chinese (n=31), followed by Malays (n=2). The age at the time of onset of melasma ranged from 25 to 64 years (mean±SD: 42.7±7.6), and the duration of disease was between three months and 33 years (mean±SD: 8.4±6.9). Other methods of skin lightening, including topical agents, lasers, IPL, and chemical peels, had been tried by 75.8 percent of the patients. There was no statistically significant difference in patient demographics between the two groups in terms of age, race, age at disease onset, duration of disease, use of sunscreen, use of topical agents, or submission to previous procedures for facial pigmentation.
The mean baseline mMASI score for the PLE group was 6.8 and for the control group was 6 (not statistically significant). At the end of the treatment, we observed a marked improvement in mMASI scores as compared with at baseline for both the placebo (44.4 %; p?0.01) and PLE (54.9%; p?0.01) groups, as it was expected due to the treatment with hydroquinone. However, at every visit, the PLE group demonstrated a greater reduction in the mMASI score than did the placebo group, which was statistically significant at Day 56 (p?0.05), whereas the placebo group experienced a 32.6-percent reduction in mMASI scores compared with 49.4 percent in the PLE group (Figure 1A). Based on the analysis of paired samples, the PLE group showed lower mMASI values than did the placebo group at every visit, reaching statistically significant differences at Day 56, V3 (p?0.05), and Day 84, V4 (p?0.05), while an important trend was already observed at Day 28, V2 (p=0.053) (Figure 1B), suggesting that the PLE group achieved a faster response to treatment than did the placebo group.
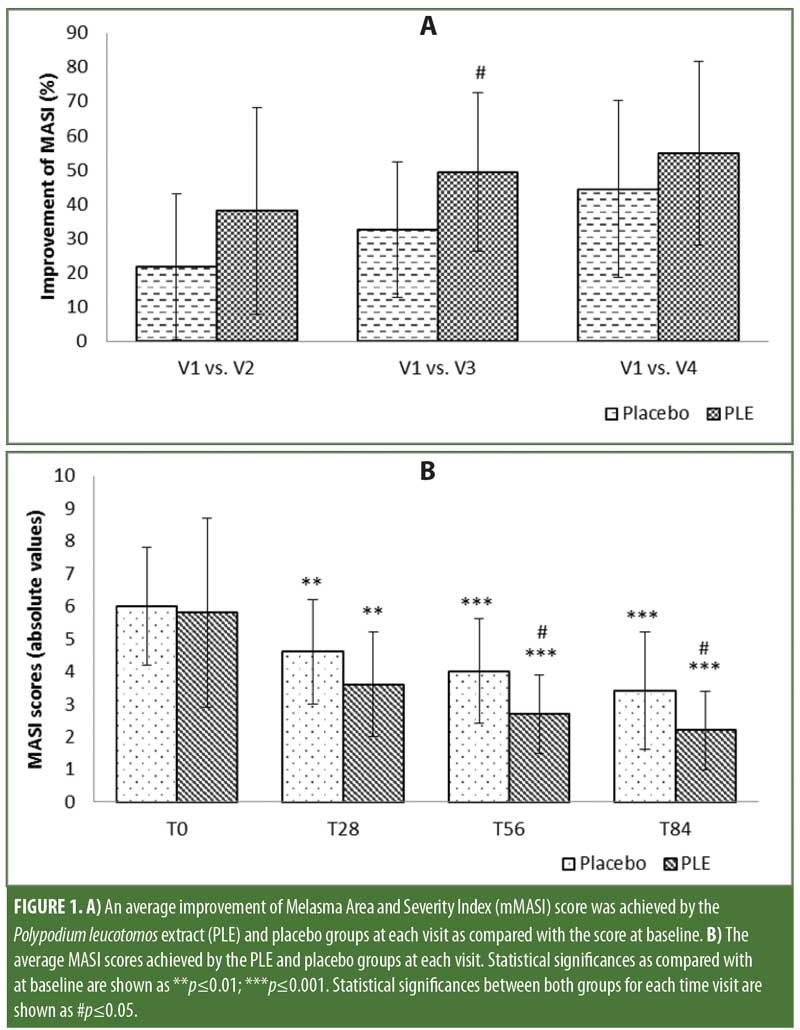
A segmentation analysis of the mMASI reduction between baseline and the end of the treatment was performed. Some degree of reduction of the mMASI score was observed in both groups. However, while 31.3 percent of the subjects in the PLE group achieved an improvement of 75 percent or greater in mMASI score, only 6.3 percent of the subjects in the placebo group achieved such a result, and this difference exhibited a statistical trend (p=0.070), which suggests that the difference might reach statistical significance if the study cohort was larger.
Further analysis showed that, after two months of treatment, 40.0 percent of the subjects of the PLE group achieved at least 60-percent improvement in mMASI score, while only 11.8 percent of the subjects in the placebo group achieved such a result (p=0.066). In the PLE group, 37.5 percent of the subjects achieved at least a 40.0-percent improvement in mMASI after only one month of treatment as compared with 17.6 percent of the subjects in the placebo group (not significant).
There was a discrete improvement in the melanin index at the end of the study as compared with baseline for both the placebo and PLE groups (15.1% and 9.7%, respectively), but the difference was not statistically significant. Similarly, the erythema index was slightly reduced in both the placebo and PLE groups at the end of the treatment (1.25% and 6.34%, respectively). Interestingly, at the third visit, the PLE group showed a significant improvement of 6.1 percent (p?0.01), while the placebo group exhibited a worsening of 2.5 percent to baseline. The difference was not statistically significant.
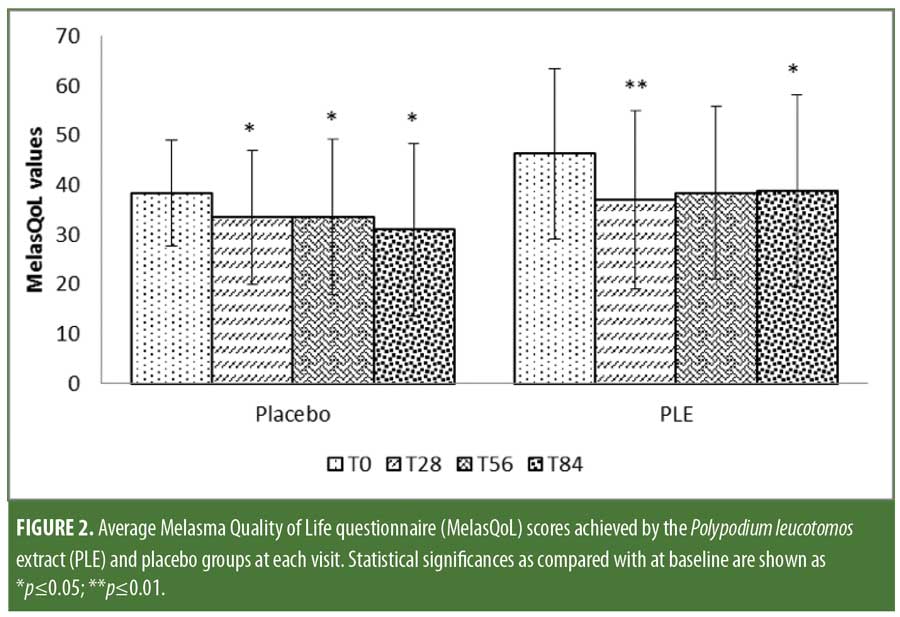
MelasQoL scores showed a marked, albeit nonsignificant, improvement throughout the study in both groups. The scores on Day 84 compared to baseline showed that the placebo group experienced an 11.7-percent improvement versus 14.9-percent improvement in the PLE group (p?0.05) (Figure 2). The PLE group displayed a four-fold decrease of MelasQoL scores as early as Day 28 as compared to that experienced by the placebo group.
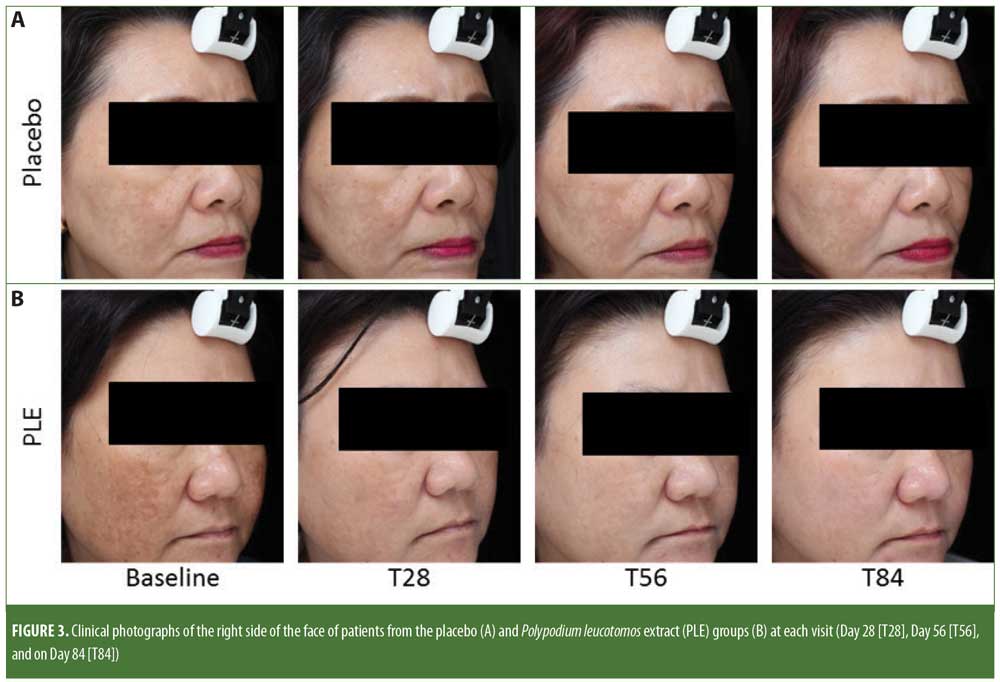
Assessment findings by the investigators based on clinical photograph observation (Figure 3) indicated an improvement at all visits for patients in both groups (PLE: p?0.01; placebo: p?0.01, respectively). Furthermore, the degree of improvement achieved between the two groups was not statistically different (p = 0.20). At Day 84, investigators reported 33-percent higher improvement of melasma in the PLE group than that of the placebo group (not significant).
There were no major side effects recorded during the study. Two patients from the PLE group and one from the placebo group reported mild itching and stinging sensation with use of the prescribed hydroquinone cream. All three patients managed to continue treatment until the completion of the study. No systemic side effects were reported.
Discussion
Oral PLE has been shown to be a promising agent for the treatment of several dermatoses, including melasma.11 It has been available as an oral supplement (Fernblock®, IFC, Madrid, Spain) in Europe since 2001 and in the United States since 2006. The PLE study product is reported to confer photoprotection; prevent against photoaging, photodermatoses, and photocarcinogenesis; and to be used as an adjuvant treatment in photo-aggravated conditions, such as melasma.18,19
A few small clinical trials have been carried out to date on PLE to demonstrate its efficacy in the treatment of melasma. To our knowledge, none have been conducted involving Asian patients. In this prospective, randomized, double-blind, placebo-controlled study, we evaluated the efficacy of oral PLE as an adjunctive treatment for melasma in this population. Overall, our findings indicate that it is an effective coadjuvant treatment for melasma in combination with topical 4% hydroquinone and sunscreen SPF 50. As expected, the PLE and placebo groups experienced improvements of their melasma, as both groups received topical 4% hydroquinone and sunscreen. However, we found a statistically significant reduction of mMASI and MelasQoL scores in the PLE group compared to those of the placebo group. In our study, subjects in the PLE group experienced significant improvement in mMASI scores from the first month of treatment, indicating that PLE accelerates the pigmentation clearance of melasma compared to those treated with topical 4% hydroquinone and sunscreen alone.
No statistically significant differences in melanin index scores were seen between the PLE and placebo groups. This might be due to limitations of the skin colorimeter in measuring pigmentary changes, by intraindividual variability of the response to treatment among the subjects, and the small cohort of this pilot study. Ahmed et al19 also failed to find a significant difference in melanin index scores between PLE and placebo groups in a cohort of Hispanic individuals receiving 240mg doses of oral PLE versus placebo three times daily for 12 weeks. However, in our study, both groups exhibited a significant improvement in melanin index scores compared to baseline, specifically a 28.8-percent reduction in the PLE group versus a more modest reduction of 13.8 percent in the placebo group (p=0.140), which indicates a clear trend of melasma amelioration by PLE among these patients. Similarly, our global assessment findings indicate greater improvement of melasma in the PLE group.
The reduction of MelasQoL score was four-fold higher at Day 28 in the PLE group versus in the placebo group, although no statistical significance was reached. Accordingly, the investigator global assessment of the photographs demonstrated improvement, though not significant, in both groups, although the PLE group achieved a 33.3-percent higher improvement in mMASI scores compared to placebo group.
The combination of improvement in MASI and MelasQoL scores and in the clinical assessment of photographs suggests that oral PLE can be used as an adjunctive treatment for melasma in combination with topical 4% hydroquinone and sunscreen. Additional research with a larger cohort and/or with a higher dose of PLE might reveal more significant results. It should be stressed that PLE should not be used in place of sun protection measures, including use of topical sunscreen and avoidance of sun exposure during the peak hours of the day. No significant side effects were reported throughout the study, confirming the safety of oral PLE.
Topically and orally administered extracts of PL have shown potential benefits in the treatment of other skin disorders, including photoaging damage resulting from UVB radiation exposure, and also have been shown to lower the incidence of UVB radiation-induced non-malignant melanoma (MM) skin cancers.23
A recent report indicated that PLE might potentially increase the MED of patients with familial melanoma compared to those with sporadic melanoma. Among patients with familial MM, those exhibiting a mutated CDKN2A and/or polymorphisms in MC1R displayed larger differences in response to treatment with PLE.24 The report also suggested that patients with familial melanoma can benefit from oral PLE, as it has been shown to significantly reduce sensitivity to UVB radiation in terms of increased MED UVB (p<0.005). Oral PLE has also shown promise in significantly reducing UVB radiation effects among patients with sporadic melanoma or dysplastic nevus syndrome, of whom UV radiation is the primary cause of melanoma genesis. Studies that include long-term follow-up and administration of PLE among patients at high risk for developing melanoma are needed to support these findings.
Additionally, a systemic photoprotective agent, such as PLE, can provide uniform, total body surface protection, which might have an advantage over topical protection.
Conclusion
Our results indicate that oral PLE (Fernblock®, IFC, Madrid, Spain) has multiple beneficial properties and a high safety profile and appears to be a useful adjunctive treatment for melasma.
Acknowledgments
The authors gratefully acknowledge Dr. Francisca Rius of the University of Málaga in Málaga, Spain, and Ms. Virlyn Tan (NSC) for for their assistance with the statistical analysis.
References
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56(4): 380–382.
- Seité S, Deshayes P, Dréno B, et al. Interest of corrective makeup in the management of patients in dermatology. Clin Cosmet Investig Dermatol. 2012;5:123–128.
- Harumi O, Goh CL. The effect of melasma on the quality of life in a sample of women living in Singapore. J Clin Aesthet Dermatol. 2016;9(1):21–24.
- Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007;20(5):308–313.
- Tan SK, Sim CS, Goh CL. Hydroquinone-induced exogenous ochronosis in Chinese–two case reports and a review. Int J Dermatol. 2008;47(6):639–640.
- Konda S, Geria AN, Halder RM. New horizons in treating disorders of hyperpigmentation in skin of color. Semin Cutan Med Surg. 2012; 31(2):133–139.
- Sarkar R, Chugh S, Garg VK. Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol. 2012; 78(4):417–428.
- Gombau L, García F, Lahoz A, et al. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol In Vitro. 2006;20(4):464–471.
- Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004; 51(6):910–918.
- Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Orally administered Polypodium leucotomos extract decreases psoralen-UVA-induced phototoxicity, pigmentation, and damage of human skin. J Am Acad Dermatol. 2004;50(1):41–49.
- Choudhry SZ, Bhatia N, Ceilley R, et al. Role of oral Polypodium leucotomos extract in dermatologic diseases: a review of the literature. J Drugs Dermatol. 2014;13(2):148–153.
- Nestor MS, Bucay V, Callender V, et al. Polypodium leucotomos as an adjunct treatment of pigmentary disorders. J Clin Aesthet Dermatol. 2014;7(3):13–17.
- González S, Gilaberte Y, Philips N, Juarranz Á. Fernblock, a nutriceutical with photoprotective properties and potential preventive agent for skin photoaging and photoinduced skin cancers. Int J Mol Sci. 2011;12(12):8466–8475.
- Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175(5):1952–1961.
- Winkelmann RR, Del Rosso J, Rigel DS. Polypodium leucotomos extract: a status report on clinical efficacy and safety. J Drugs Dermatol. 2015;14(3):254–261.
- Murbach TS, Béres E, Vértesi A, et al. A comprehensive toxicological safety assessment of an aqueous extract of Polypodium leucotomos (Fernblock®). Food Chem Toxicol. 2015;86:328–341.
- Nestor MS, Berman B, Swenson N. Safety and efficacy of oral Polypodium leucotomos extract in healthy adult subjects. J Clin Aesthet Dermatol. 2015;8(2):19–23.
- Schalka S, Vitale-Villarejo MA, Monteiro-Agelune C, Pinto-Bombarda PC. The benefits of using a compound containing Polypodium leucotomos extract for reducing erythema and pigmentation resulting from ultraviolet radiation. Surg Cosmet Dermatol. 2014;6(4):344–348.
- Martin LK, Caperton C, Woolery-Lloyd H, et al. A randomized double-blind placebo controlled study evaluating the effectiveness and tolerability of oral Polypodium leucotomos in patients with melasma. American Academy of Dermatology Annual Meeting. San Diego, CA; March 16–20, 2012.
- Ahmed AM, López I, Perese F, et al. A randomized, double-blinded, placebo-controlled trial of oral Polypodium leucotomos extract as an adjunct to sunscreen in the treatment of melasma. JAMA Dermatol. 2013;149(8):981–983.
- Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modi?ed MASI scoring method. J Am Acad Dermatol. 2009;64(1):78–83.
- Balkrishnan R, McMichael AJ, Camacho FT, et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149(3):572–577.
- Alcaraz MV, Pathak MA, Rius F, et al. An extract of Polypodium leucotomos appears to minimize certain photoaging changes in a hairless albino mouse animal model. A pilot study. Photodermatol Photoimmunol Photomed. 1999;15(3–4):120–126.
- Aguilera P, Carrera C, Puig-Butille JA, et al. Benefits of oral Polypodium leucotomos extract in MM high-risk patients. J Eur Acad Dermatol Venereol. 2013;27(9): 1095–1100.

